On October 21, 2022, Lauren Lempert presented at one of the Regan-Udall Foundation Expert Panels listening sessions on how to improve FDA operations of FDA’s Center for Tobacco Products operations. She and I followed up with a written comment letter detailing many of the problems with the tobacco product application processes, including both the premarketContinue reading “Recommendations to improve FDA premarket tobacco product application review to follow statutory mandates and improve scientific rigor”
Category Archives: tobacco companies
Kids more addicted to e-cigs since Juul introduced protonated nicotine
One of the big innovations Juul pioneered was adding acid to the liquid in e-cigarettes to make the nicotine aerosol less alkaline and easier to inhale. This so-called protonated nicotine allows the e-cigarettes to deliver higher doses of the addictive drug nicotine. Therefore, we would expect to see increasing measures of addiction among e-cigarette usingContinue reading “Kids more addicted to e-cigs since Juul introduced protonated nicotine”
Regan-Udall Foundation review of FDA tobacco regulation “listening sessions” dominated by pro-industry speakers
Regan-Udall Foundation review of FDA tobacco regulation “listening sessions” dominated by pro-industry speakers The FDA has asked the Regan-Udall Foundation for recommendations on how to improve tobacco product regulation. The Foundation’s Tobacco Independent Expert Panel has had three “listening sessions” that have been dominated by industry players and “harm reduction” advocates. I have previously expressedContinue reading “Regan-Udall Foundation review of FDA tobacco regulation “listening sessions” dominated by pro-industry speakers“
New report explains menthol marketing from the beginning and why we need to end it
Robert Jackler and his colleagues at the Stanford Research Into the Impact of Tobacco Advertising recently released “ADVERTISING CREATED & CONTINUES TO DRIVE THE MENTHOL TOBACCO MARKET: Methods Used by The Industry to Target Youth, Women, & Black Americans,” an encyclopedic history of menthol marketing in the United States. Jackler and his colleagues complement aContinue reading “New report explains menthol marketing from the beginning and why we need to end it”
33 state Juul settlement does not seem to have fixed problems with earlier settlements
On September 6, 2022, Connecticut Attorney General AG Tong announced that 33 states and Puerto Rico who were suing Juul for its predatory marketing had reached a settlement in principle. Tong’s press release stated, “Both the financial and injunctive terms exceed any prior agreement JUUL has reached with states to date.” While the devil isContinue reading “33 state Juul settlement does not seem to have fixed problems with earlier settlements”
Cannabis companies engage in “corporate social responsibility” activities similar to what tobacco companies do
The tobacco companies (and some other companies) use “corporate social responsibility” programs in which the companies stress some socially desirable behavior — such as affirmative action in hiring or supporting cultural institutions — to distract attention from the fact that they engage in predatory marketing of a dangerous product. As the legal cannabis industry emerges,Continue reading “Cannabis companies engage in “corporate social responsibility” activities similar to what tobacco companies do”
Trump judge continues to delay cigarette warning labels by doing nothing
Last May a federal judge in Texas appointed by President Trump again delayed ruling on the tobacco companies’ lawsuit against the Congressionally-mandated warnings on cigarette packs that the FDA finally issued in 2020. Now he has done it again, pushing the rule’s effective date for the warning labels by an additional 90 days, to OctoberContinue reading “Trump judge continues to delay cigarette warning labels by doing nothing”
Another way FDA is cutting ecig companies slack: shelf life
E-cigarettes have finite shelf lives because they become more toxic over time while they sit on the shelf. E-cigarettes are often contaminated with bacteria and fungus that grow while the e-cigarettes sit on the shelf. In addition, the chemicals in the e-liquid continue reacting with each other after they are mixed, growing more toxic overContinue reading “Another way FDA is cutting ecig companies slack: shelf life”
Juul knows why FDA denied its request to legally sell its ecigs. Why won’t FDA tell the rest of us?
On June 23, 2022, FDA denied Juul’s application for authorization to sell its e-cigarettes in the USA. While FDA quickly backed down and allowed Juul to remain on the market while the FDA considered “scientific issues unique to the JUUL application that warrant additional review,” there is a lot of interest in FDA’s reasoning forContinue reading “Juul knows why FDA denied its request to legally sell its ecigs. Why won’t FDA tell the rest of us?”
FDA should not grant exemptions to its proposed standard banning menthol in cigarettes that would continue to allow menthol heated tobacco products or low nicotine cigarettes
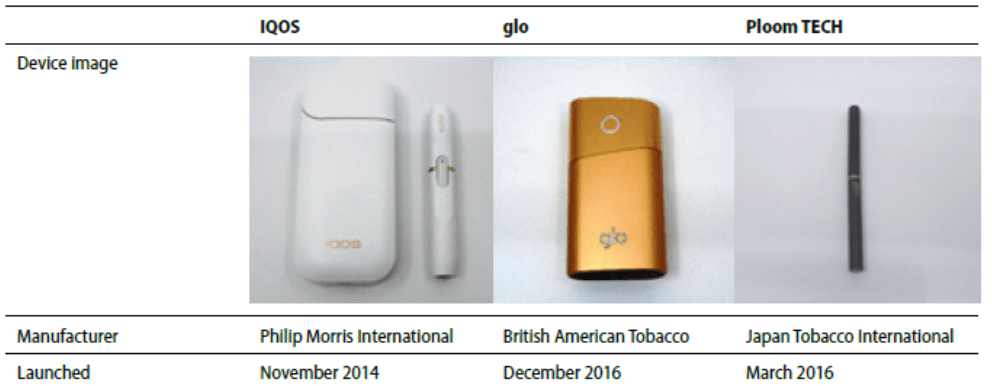
FDA’s proposed standard banning menthol in cigarettes is very well-done except for its provision that would allow FDA to exempt heated tobacco products like Philip Morris’ IQOS, BAT’s glo or JTI’s Ploom — which are considered “cigarettes” under the law — or low nicotine cigarettes. (FDA has only authorized sale IQOS so far.) This isContinue reading “FDA should not grant exemptions to its proposed standard banning menthol in cigarettes that would continue to allow menthol heated tobacco products or low nicotine cigarettes”