Huiliang Qiu, Matt Springer, and colleagues’ paper “Increased vulnerability to atrial and ventricular arrhythmias caused by different types of inhaled tobacco or marijuana products” makes two important points: (1) even very short daily exposures to tobacco and marijuana products have serious cumulative effects on the heart, and (2) nicotine and THC, the psychoactive components ofContinue reading “Using e-cigs, cigs, heated tobacco products, or marijuana once a day increases risk of heart arrythmias and causes remodeling of the heart“
Category Archives: smoking
One reason why dual use of ecigs and cigs is worse than using either product alone
Once upon a time, people – including me – thought eicgs were just cigarettes without the combustion, so they had to be less toxic. By this logic, dual users (people who used e-cigs and cigs at the same time) were probably no worse off than smokers. Now we know that is wrong. While they doContinue reading “One reason why dual use of ecigs and cigs is worse than using either product alone”
FDA pulls some menthol e-cigs off the market: What about the rest?
Last week FDA took the important step of denying permission for Logic Technology to market is menthol e-cigarettes. In its press release, FDA specifically recognized the attraction of menthol e-cigarettes to youth: For non-tobacco-flavored e-cigarettes, including menthol-flavored e-cigarettes, existing evidence demonstrates a known and substantial risk with regard to youth appeal, uptake and use. RecentContinue reading “FDA pulls some menthol e-cigs off the market: What about the rest?”
New report explains menthol marketing from the beginning and why we need to end it
Robert Jackler and his colleagues at the Stanford Research Into the Impact of Tobacco Advertising recently released “ADVERTISING CREATED & CONTINUES TO DRIVE THE MENTHOL TOBACCO MARKET: Methods Used by The Industry to Target Youth, Women, & Black Americans,” an encyclopedic history of menthol marketing in the United States. Jackler and his colleagues complement aContinue reading “New report explains menthol marketing from the beginning and why we need to end it”
1.7 million more kids started smoking above historical trends after ecigs became widely used
Melissa Harrell and her colleagues just published important quantitative information adding to the case that e-cigs are promoting rather than replacing cigarette smoking among youth. Their new paper, “Impact of the e-cigarette era on cigarette smoking among youth in the United States: A population-level study,” estimates changes in youth smoking prevalence after 2014 when e-cigContinue reading “1.7 million more kids started smoking above historical trends after ecigs became widely used”
Thai kids who start with e-cigs more likely to go on to add cigs
Kade Patanavanich, Methavee Worawattanakul, and I just published “Longitudinal bidirectional association between youth electronic cigarette use and tobacco cigarette smoking initiation in Thailand” in Tobacco Control. This paper shows that in Thailand, as in richer countries, never-smoking youth who use e-cigarettes are more likely to become smokers than kids who don’t use e-cigarettes. (Kids whoContinue reading “Thai kids who start with e-cigs more likely to go on to add cigs”
Trump judge continues to delay cigarette warning labels by doing nothing
Last May a federal judge in Texas appointed by President Trump again delayed ruling on the tobacco companies’ lawsuit against the Congressionally-mandated warnings on cigarette packs that the FDA finally issued in 2020. Now he has done it again, pushing the rule’s effective date for the warning labels by an additional 90 days, to OctoberContinue reading “Trump judge continues to delay cigarette warning labels by doing nothing”
FDA should not grant exemptions to its proposed standard banning menthol in cigarettes that would continue to allow menthol heated tobacco products or low nicotine cigarettes
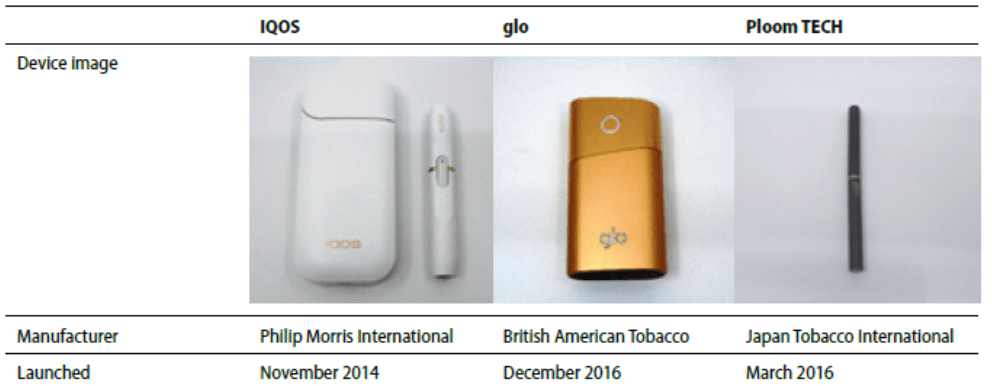
FDA’s proposed standard banning menthol in cigarettes is very well-done except for its provision that would allow FDA to exempt heated tobacco products like Philip Morris’ IQOS, BAT’s glo or JTI’s Ploom — which are considered “cigarettes” under the law — or low nicotine cigarettes. (FDA has only authorized sale IQOS so far.) This isContinue reading “FDA should not grant exemptions to its proposed standard banning menthol in cigarettes that would continue to allow menthol heated tobacco products or low nicotine cigarettes”
FDA should prohibit all menthol flavor additives, compounds, constituents, and ingredients in cigarettes, and should not limit the proposed standard to prohibiting menthol as a “characterizing flavor”
My colleagues at UCSF and other universities and I have submitted this comment to the FDA in response to its proposal to prohibit the use of menthol in cigarettes. A PDF of the comment is here. The Regulations.gov tracking number is l6b-ew3y-c1ye. FDA should prohibit all menthol flavor additives, compounds, constituents, and ingredients in cigarettes,Continue reading “FDA should prohibit all menthol flavor additives, compounds, constituents, and ingredients in cigarettes, and should not limit the proposed standard to prohibiting menthol as a “characterizing flavor””
Cutting phase-in for cig menthol ban from 1 year to 90 days will prevent 265,000 kids from smoking and 12,000 premature deaths
FDA’s draft rule ending menthol cigarettes proposes giving tobacco companies a year to clear menthol cigarettes off the market. FDA requested public comment on shortening the phase in 90 days. My UCSF and Stanford colleagues and I submitted this public comment (PDF version) supporting a 90 day phase in. We noted that FDA’s own analysisContinue reading “Cutting phase-in for cig menthol ban from 1 year to 90 days will prevent 265,000 kids from smoking and 12,000 premature deaths”