While many organizations and people contributed to the FDA finally issuing a draft rule to get rid of menthol in cigarettes and flavors in cigars, the one at the top of my list is the African American Tobacco Control Leadership Council and the “three musketeers of menthol,” Valerie Yerger, Carol McGruder and Phil Gardiner. ThisContinue reading “We should all thank the African American Tobacco Control Leadership Council for the FDA menthol rule”
Category Archives: FDA
FDA authorizes NJOY e-cigs without disclosing reasoning; inaction allows menthol to remain on the market
Today FDA continued its trend of authorizing the sale of tobacco-flavored eicgs — this time the NJOY while leaving menthol versions on the market by not acting. On the NJOY website, menthol and tobacco pods are sold side-by-side, with menthol shown first (screen shot below). Following the precedent set when it authorized the sale ofContinue reading “FDA authorizes NJOY e-cigs without disclosing reasoning; inaction allows menthol to remain on the market”
FDA drags its feet on releasing scientific justification for authorizing Logic e-cigs and HTP
As I described before, FDA broke precedent by not releasing the full Technical Project Lead (TPL) reports summarizing its positive decisions on 8 electronic nicotine delivery system (ENDS) products made by Logic Technologies. Instead, it just released 4 of the 56 pages, most of which constituted uninformative boilerplate. There is no excuse for withholding thisContinue reading “FDA drags its feet on releasing scientific justification for authorizing Logic e-cigs and HTP”
Why is FDA withholding details of its approval for 8 Logic e-cigs and HTP?
In the past, when FDA announced that it was authorizing the sale of new products through the Premarket Tobacco Authorization process (PMTA), they released a detailed scientific summary of the rationale behind their decision known as the Technical Project Lead (TPL) report. These documents were released on the FDA web page announcing the positive decisions.Continue reading “Why is FDA withholding details of its approval for 8 Logic e-cigs and HTP?”
FDA sends another signal that it will authorize menthol ecigs despite high youth use
The FDA continues to telegraph that it is going to authorize the sale of menthol e-cigarettes. The first strong indication was not acting on the Vuse Solo menthol PMTA, which has allowed RJ Reynolds to continue selling menthol Vuse side-by-side (screen capture below) with the authorized tobacco flavor. Now, the FDA press release announcing itsContinue reading “FDA sends another signal that it will authorize menthol ecigs despite high youth use”
E-cigarettes more likely to lead to ongoing nicotine addiction than NRT
E-cigarette advocates and, more important, the FDA, takes it as given that e-cigarettes were developed as a harm reduction tool to help smokers quit cigarettes by “switching completely.” This assumption is, however, inconsistent with the tobacco companies’ actual reasons for developing e-cigarettes and other non-combustible products, including tobacco company products approved as NRT: To deterContinue reading “E-cigarettes more likely to lead to ongoing nicotine addiction than NRT”
Higher nicotine content and flavors increase e-cig abuse liability
As debates over nicotine and flavors in e-cigarettes continues to heat up, Mari Gades, Aleksandra Alcheva, Amy Riegelman, and Dorothy Hatsukami just published “The role of nicotine and flavor in the abuse potential and appeal of electronic cigarettes for adult current and former cigarette and electronic cigarette users: A systematic review” in Nicotine and TobaccoContinue reading “Higher nicotine content and flavors increase e-cig abuse liability”
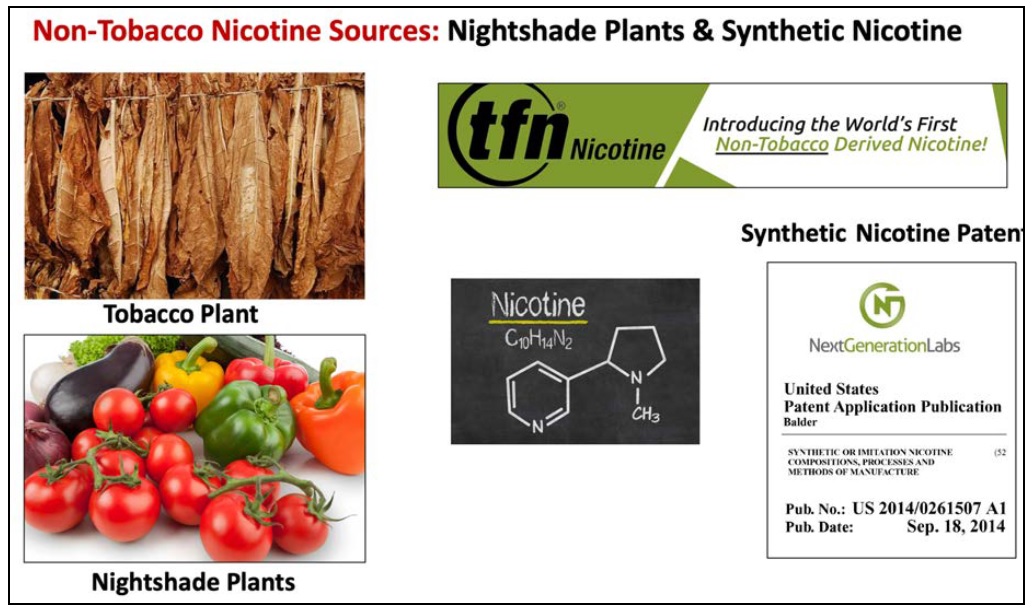
New report highlights marketing of synthetic and “non-tobacco” nicotine products
Just as Congress is moving toward giving FDA explicit authority over non-tobacco nicotine products, Rob Jackler’s group at Stanford released a new report, “Marketing of ‘Tobacco-Free’ and ‘Synthetic Nicotine’ Products,” that details how these products are being promoted. In 2009 Congress gave the FDA jurisdiction over consumer products “made or derived from tobacco,” which includesContinue reading “New report highlights marketing of synthetic and “non-tobacco” nicotine products”
Proposed FDA menthol rules sent to Biden OMB for approval before release for public comment
One February 24, 2022, the FDA submitted two proposed rules on menthol to the White House Office of Management and Budget for review before public release: “Tobacco Product Standard for Menthol in Cigarettes” and “Tobacco Product Standard for Characterizing Flavors in Cigars.” The OMB can approve the proposed rules as submitted, deny them, or approveContinue reading “Proposed FDA menthol rules sent to Biden OMB for approval before release for public comment”
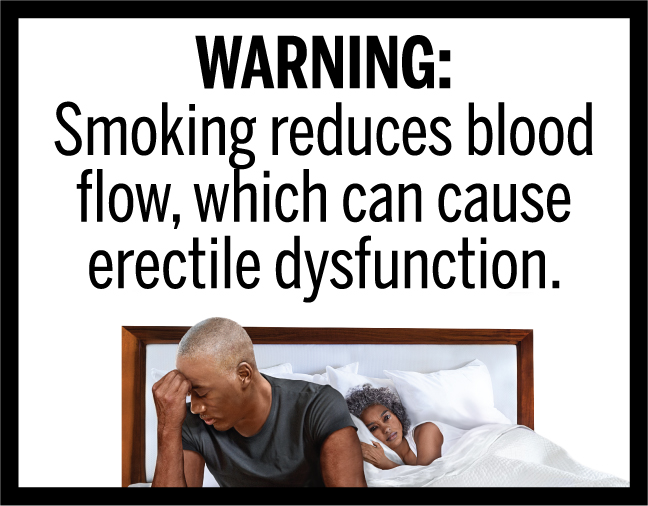
Court inaction continues to block Congressionally-mandated graphic warning labels on cigs
The 2009 Family Smoking Prevention and Tobacco Control Act directed FDA to issue a rule requiring state-of-the-art graphic warning labels on cigarette packages. FDA issued such a rule, but the tobacco companies sued and blocked the original warnings in court in 2013. It took years, but in March 2020 FDA issued a new carefully designedContinue reading “Court inaction continues to block Congressionally-mandated graphic warning labels on cigs”