FDA’s draft rule ending menthol cigarettes proposes giving tobacco companies a year to clear menthol cigarettes off the market. FDA requested public comment on shortening the phase in 90 days. My UCSF and Stanford colleagues and I submitted this public comment (PDF version) supporting a 90 day phase in. We noted that FDA’s own analysisContinue reading “Cutting phase-in for cig menthol ban from 1 year to 90 days will prevent 265,000 kids from smoking and 12,000 premature deaths”
Category Archives: youth
FDA granting the tobacco companies’ request to extend the public comment period its menthol cigarette ban will lead to 58,000 more smokers and 2,700 more deaths
FDA has invited public comment on its well-justified rule to get rid of menthol cigarettes. Even though dealing with menthol cigarettes has been being considered and debated for 13 years, the tobacco companies have told FDA they need more time to prepare their comments and asked that the comment period be extended for 60 days.Continue reading “FDA granting the tobacco companies’ request to extend the public comment period its menthol cigarette ban will lead to 58,000 more smokers and 2,700 more deaths”
Direct evidence that Juul increases nicotine addiction, especially among kids
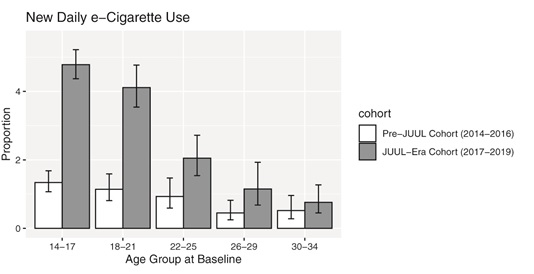
John Pierce and his colleagues at UCSD just published “Daily E-cigarette Use and the Surge in JUUL Sales: 2017-2019” that shows shows that after Juul came on the market there was a 3.6-fold higher rate of progression to daily e-cigarette use — a marker of addiction — among 14-17 year olds compared to 3 yearsContinue reading “Direct evidence that Juul increases nicotine addiction, especially among kids”
FDA prejudged e-cigs as good before it evaluated a single application
An August 31, 2020 memo detailing FDA’s plan for dealing with the expected flood of Premarket Tobacco Applications (PMTAs), clearly prejudges e-cigarettes as a good thing before looking at a single application. In particular, the FDA describes its “public heath goals” as including Ensure a variety of ENDS [electronic nicotine delivery systems] have an opportunityContinue reading “FDA prejudged e-cigs as good before it evaluated a single application”
FDA does JT/Logic’s work for it to justify authorizing 3 of its ENDS
On March 24, 2022 FDA authorized the sale of three electronic nicotine delivery systems distributed by Japan Tobacco’s US company Logic Technology Development, – two conventional e-cigarettes and one heated tobacco product. FDA withheld the Technical Project Lead (TPL) report that provides the scientific justifications for its decision. Thanks to my colleague Lauren Lempert filingContinue reading “FDA does JT/Logic’s work for it to justify authorizing 3 of its ENDS”
FDA’s proposed ban on menthol cigarettes explains why FDA needs to reverse its de facto approvals of menthol e-cigarettes
The FDA’s decisions to act on premarket tobacco product applications (PMTAs) for e-cigarettes has been explicit about two points: (1) it is authorizing tobacco flavors because kids don’t seem to use tobacco flavored e-cigs and (2) it is blocking flavored e-cigs – except menthol – because kids like flavors. Because they can’t justify allowing mentholContinue reading “FDA’s proposed ban on menthol cigarettes explains why FDA needs to reverse its de facto approvals of menthol e-cigarettes”
FDA authorizes two more RJR tobacco e-cigs and lets menthol remain on the market, ignoring its own conclusion that menthol reinforces nicotine addiction in kids’ developing brains
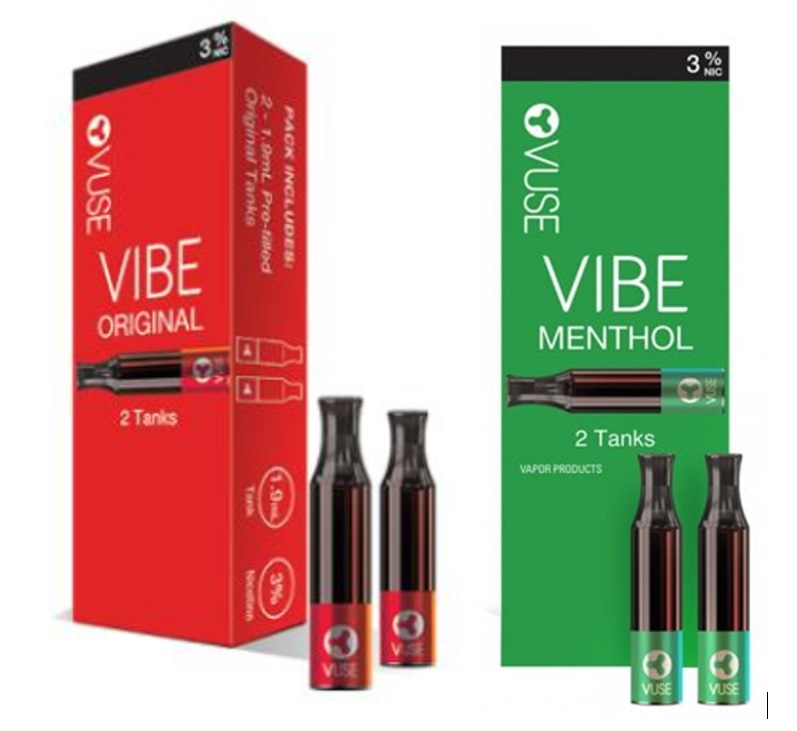
Today (May 12, 2022) FDA announced that it was authorizing the sale of two additional RJR e-cigarettes, Vuse Vibe (a vape pen) and Vuse Ciro (similar to previously authorized Vuse Solo). FDA didn’t act on RJR’s most popular Vuse product, Vuse Alto, so it continues on the market. Following FDA’s de facto policy, both wereContinue reading “FDA authorizes two more RJR tobacco e-cigs and lets menthol remain on the market, ignoring its own conclusion that menthol reinforces nicotine addiction in kids’ developing brains”
Ending the sale of flavored tobacco products is the real harm reduction
Yesterday (May 9, 2022) I testified before the Colorado Senate Finance Committee supporting a proposed statewide ban on the sale of all flavored tobacco products. As usual, opponents claimed that flavored products were needed for “harm reduction.” In listening to their testimony, I realized that for e-cigs and other tobacco products to actually reduce harm,Continue reading “Ending the sale of flavored tobacco products is the real harm reduction”
A Juul settlement that would actually protect kids
The first of the private lawsuits against Juul is scheduled to go to trial next month in San Francisco. The whole legal system is designed to encourage voluntary settlement, so it is likely that there are or soon will be serious negotiations between the plaintiffs and Juul in an effort to avoid the risks thatContinue reading “A Juul settlement that would actually protect kids”
FDA proposed menthol ban in cigarettes is well done, except for the exceptions
On April 28, 2022, FDA released its proposed tobacco product standard prohibiting menthol cigarettes. The justification for prohibiting menthol as a characterizing flavor includes a comprehensive review of the effects of menthol on smoking, including why menthol promotes cigarette initiation and how it makes it harder to quit smoking. In addition to summarizing the behavioralContinue reading “FDA proposed menthol ban in cigarettes is well done, except for the exceptions”