Perhaps the most important provision in the settlement between the State of North Carolina and Juul involved the release of previously secret internal Juul documents. These documents are particularly timely given the fact that Juul and the FDA are now fighting over FDA’s decision not to authorize the same of Juul e-cigarettes as well asContinue reading “Where are the Juul documents?”
Category Archives: tobacco companies
FDA science leader Matt Holman leaves FDA for PMI
Matt Holman, who has served as head of the FDA Center for Tobacco Products since 2017, is leaving the FDA to take a job with Philip Morris International. According to a story in The Hill, a spokesperson for Philip Morris said Holman “is committed to helping existing adult smokers access scientifically substantiated smoke-free alternatives whileContinue reading “FDA science leader Matt Holman leaves FDA for PMI”
Since FDA has made a mess of Juul, the health community & Congress should force FDA to ban menthol e-cigs now
The day after FDA denied Juul’s application to continue selling its e-cigarettes on June 23, 2022, Juul sued and obtained an emergency Stay from the US Court of Appeals. On July 5, the FDA announced that it was issuing its own Administrative Stay and allowed Juul to remain on the market while the FDA considersContinue reading “Since FDA has made a mess of Juul, the health community & Congress should force FDA to ban menthol e-cigs now”
White House OKs FDA moving forward on reducing nicotine in cigs; FDA can and should ban menthol ecigs now
On June 21, 2022, the Biden Administration announced that FDA would be moving forward to develop a product standard limiting nicotine in cigarettes and other combusted tobacco products to nonaddictive levels. FDA has been formally considering such a rule for a long time. In 2018, FDA solicited public comment on such a rule. At thatContinue reading “White House OKs FDA moving forward on reducing nicotine in cigs; FDA can and should ban menthol ecigs now”
FDA should finalize the proposed rule prohibiting characterizing flavors in cigars and make it effective 90 days after publication of the final rule
My colleagues and I submitted this public comment to FDA urging them not to extend the comment period for the rule ending flavored cigars, as the tobacco companies have requested. Using the FDA’s own analysis cutting the shortening the effective date by nine months (from one-year to 90-days) would prevent an additional 396,000 people fromContinue reading “FDA should finalize the proposed rule prohibiting characterizing flavors in cigars and make it effective 90 days after publication of the final rule”
FDA should not give the tobacco companies more time to submit public comments about the proposed rule ending characterizing flavors in cigars
My colleagues and I submitted this public comment to FDA urging them not to extend the comment period for the rule ending flavored cigars, as the tobacco companies have requested. According to the FDA’s own analysis granting the 3 month delay would cost 60 lives and an additional $1.17-$1.43 billion in costs. The industry hasContinue reading “FDA should not give the tobacco companies more time to submit public comments about the proposed rule ending characterizing flavors in cigars”
FDA granting the tobacco companies’ request to extend the public comment period its menthol cigarette ban will lead to 58,000 more smokers and 2,700 more deaths
FDA has invited public comment on its well-justified rule to get rid of menthol cigarettes. Even though dealing with menthol cigarettes has been being considered and debated for 13 years, the tobacco companies have told FDA they need more time to prepare their comments and asked that the comment period be extended for 60 days.Continue reading “FDA granting the tobacco companies’ request to extend the public comment period its menthol cigarette ban will lead to 58,000 more smokers and 2,700 more deaths”
Direct evidence that Juul increases nicotine addiction, especially among kids
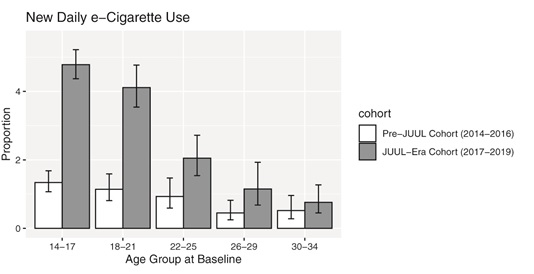
John Pierce and his colleagues at UCSD just published “Daily E-cigarette Use and the Surge in JUUL Sales: 2017-2019” that shows shows that after Juul came on the market there was a 3.6-fold higher rate of progression to daily e-cigarette use — a marker of addiction — among 14-17 year olds compared to 3 yearsContinue reading “Direct evidence that Juul increases nicotine addiction, especially among kids”
FDA prejudged e-cigs as good before it evaluated a single application
An August 31, 2020 memo detailing FDA’s plan for dealing with the expected flood of Premarket Tobacco Applications (PMTAs), clearly prejudges e-cigarettes as a good thing before looking at a single application. In particular, the FDA describes its “public heath goals” as including Ensure a variety of ENDS [electronic nicotine delivery systems] have an opportunityContinue reading “FDA prejudged e-cigs as good before it evaluated a single application”
FDA does JT/Logic’s work for it to justify authorizing 3 of its ENDS
On March 24, 2022 FDA authorized the sale of three electronic nicotine delivery systems distributed by Japan Tobacco’s US company Logic Technology Development, – two conventional e-cigarettes and one heated tobacco product. FDA withheld the Technical Project Lead (TPL) report that provides the scientific justifications for its decision. Thanks to my colleague Lauren Lempert filingContinue reading “FDA does JT/Logic’s work for it to justify authorizing 3 of its ENDS”