My colleagues at the UCSF TCORS and I submitted this public comment to the FDA on its proposal to remove flavors from cigars. A PDF of the comment is here. The Regulations.gov tracking number is l6c-lwj1-vcu7. FDA should extend the scope of the proposed flavor standard to prohibit flavors in all tobacco products, including e-cigarettesContinue reading “FDA should extend the scope of its proposed cigar flavor standard to prohibit flavors in all tobacco products, including e-cigarettes and smokeless tobacco, to make them less appealing to adolescents and young adults”
Category Archives: FDA
FDA should not grant exemptions to its proposed standard banning menthol in cigarettes that would continue to allow menthol heated tobacco products or low nicotine cigarettes
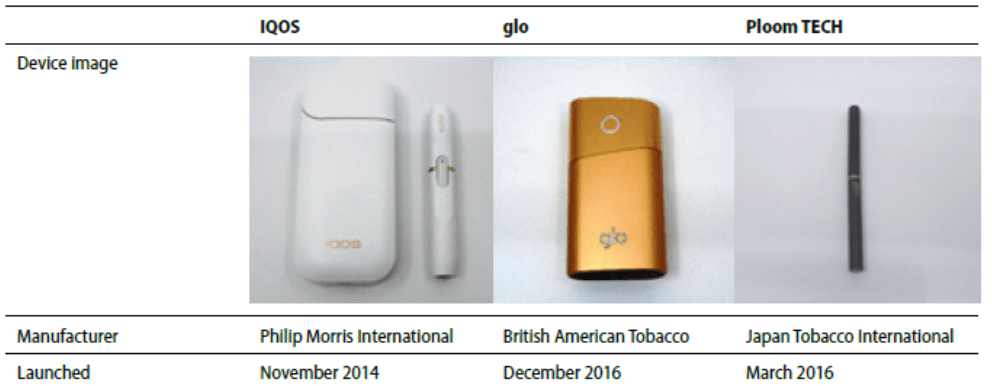
FDA’s proposed standard banning menthol in cigarettes is very well-done except for its provision that would allow FDA to exempt heated tobacco products like Philip Morris’ IQOS, BAT’s glo or JTI’s Ploom — which are considered “cigarettes” under the law — or low nicotine cigarettes. (FDA has only authorized sale IQOS so far.) This isContinue reading “FDA should not grant exemptions to its proposed standard banning menthol in cigarettes that would continue to allow menthol heated tobacco products or low nicotine cigarettes”
FDA should prohibit all menthol flavor additives, compounds, constituents, and ingredients in cigarettes, and should not limit the proposed standard to prohibiting menthol as a “characterizing flavor”
My colleagues at UCSF and other universities and I have submitted this comment to the FDA in response to its proposal to prohibit the use of menthol in cigarettes. A PDF of the comment is here. The Regulations.gov tracking number is l6b-ew3y-c1ye. FDA should prohibit all menthol flavor additives, compounds, constituents, and ingredients in cigarettes,Continue reading “FDA should prohibit all menthol flavor additives, compounds, constituents, and ingredients in cigarettes, and should not limit the proposed standard to prohibiting menthol as a “characterizing flavor””
FDA science leader Matt Holman leaves FDA for PMI
Matt Holman, who has served as head of the FDA Center for Tobacco Products since 2017, is leaving the FDA to take a job with Philip Morris International. According to a story in The Hill, a spokesperson for Philip Morris said Holman “is committed to helping existing adult smokers access scientifically substantiated smoke-free alternatives whileContinue reading “FDA science leader Matt Holman leaves FDA for PMI”
Outside review of FDA CTP operations brings opportunities and risks
FDA Commissioner Rob Califf announced (Politico, Associated Press, New York Times) that he was asking the Regan-Udall Foundation to conduct a process review of the FDA’s tobacco and food centers. As someone who has been critical of the poor and incomplete scientific assessments FDA has completed of so-called “harm reduction” products — particularly e-cigarettes andContinue reading “Outside review of FDA CTP operations brings opportunities and risks”
Since FDA has made a mess of Juul, the health community & Congress should force FDA to ban menthol e-cigs now
The day after FDA denied Juul’s application to continue selling its e-cigarettes on June 23, 2022, Juul sued and obtained an emergency Stay from the US Court of Appeals. On July 5, the FDA announced that it was issuing its own Administrative Stay and allowed Juul to remain on the market while the FDA considersContinue reading “Since FDA has made a mess of Juul, the health community & Congress should force FDA to ban menthol e-cigs now”
As a matter of health equity and social justice, FDA should immediately finalize and implement the proposed standard for menthol in cigarettes to reduce smoking-attributable deaths and health disparities among African Americans
Several colleagues at UCSF and elsewhere and I submitted this comment to the FDA on its proposed rule to end menthol in cigarettes. A PDF is here; the regulations.gov tracking number is l4s-xj6c-9kba. As a matter of health equity and social justice, FDA should immediately finalize and implement the proposed standard for menthol in cigarettesContinue reading “As a matter of health equity and social justice, FDA should immediately finalize and implement the proposed standard for menthol in cigarettes to reduce smoking-attributable deaths and health disparities among African Americans”
White House OKs FDA moving forward on reducing nicotine in cigs; FDA can and should ban menthol ecigs now
On June 21, 2022, the Biden Administration announced that FDA would be moving forward to develop a product standard limiting nicotine in cigarettes and other combusted tobacco products to nonaddictive levels. FDA has been formally considering such a rule for a long time. In 2018, FDA solicited public comment on such a rule. At thatContinue reading “White House OKs FDA moving forward on reducing nicotine in cigs; FDA can and should ban menthol ecigs now”
FDA should finalize the proposed rule prohibiting characterizing flavors in cigars and make it effective 90 days after publication of the final rule
My colleagues and I submitted this public comment to FDA urging them not to extend the comment period for the rule ending flavored cigars, as the tobacco companies have requested. Using the FDA’s own analysis cutting the shortening the effective date by nine months (from one-year to 90-days) would prevent an additional 396,000 people fromContinue reading “FDA should finalize the proposed rule prohibiting characterizing flavors in cigars and make it effective 90 days after publication of the final rule”
FDA should not give the tobacco companies more time to submit public comments about the proposed rule ending characterizing flavors in cigars
My colleagues and I submitted this public comment to FDA urging them not to extend the comment period for the rule ending flavored cigars, as the tobacco companies have requested. According to the FDA’s own analysis granting the 3 month delay would cost 60 lives and an additional $1.17-$1.43 billion in costs. The industry hasContinue reading “FDA should not give the tobacco companies more time to submit public comments about the proposed rule ending characterizing flavors in cigars”