E-cigarette advocates, including the National Health Service in England, promote e-cigarette use to pregnant women as an alternative to smoking cigarettes. When we conducted our systematic review and meta-analysis of the association between e-cigarette use and disease in people, there were not enough studies of effects on pregnancy to do a quantitative risk estimate. The few available studies, however, did not report adverse effects.
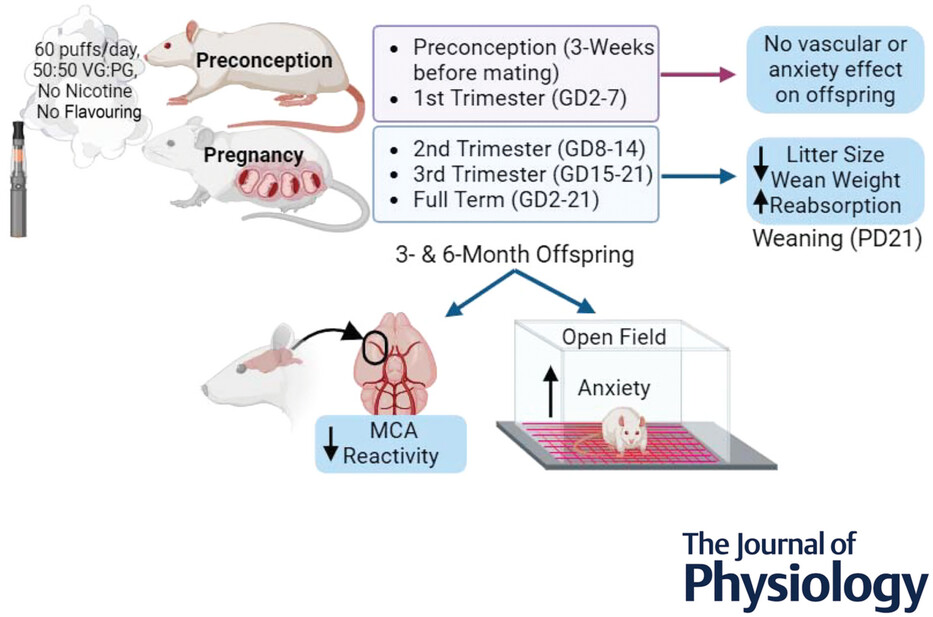
Amber Mills and colleagues new paper Influence of gestational window on offspring vascular health in rodents with in utero exposure to electronic cigarettes, however, provides strong experimental evidence (in rats) that exposure to e-cigarette aerosol during pregnancy reduces the number and size of pups, adversely affects development of blood vessels, as well as pups’ behavior after birth.
They found that maternal exposure to just 60-puffs/day (5 days/week) in the second and third trimesters led to increased pup loss (uterine reabsorption), changes in body composition, body mass, and pup deaths after birth. The ability of an artery in the brain to dilate in response to increased need for blood flow was reduced by about on-third. Pups whose mothers were exposed to e-cigarette aerosol while pregnant also exhibited more anxiety-like behavior after birth, indicating that e-cigarette exposure while pregnant influences offsprings’ neurodevelopment and metabolism.
Exposure of the mothers before pregnancy did not have any of these effects, showing that it is exposure among pregnancy, particularly the second and third trimester, that are adversely affecting fetal development.
Mills and colleagues also have an excellent discussion of the literature shows that their results are consistent with the emerging evidence — largely from other animal studies — that e-cigarette exposure during pregnancy has a wide range of adverse effects on the developing fetus.
PG/VG caused this damage
Significantly, the e-cigarette aerosol was from nicotine-free e-cigarettes, consisting only of propylene glycol and vegetable glycerine (PG/VG), adding to the evidence that PG/VG is itself dangerous. (The NHS assumes that e-cigarettes are less dangerous than cigarettes because they don’t deliver carbon monoxide; they don’t mention PG/VG.)
This study also adds to the evidence that the FDA was on the right track in 2019 when it proposed updating its list of Harmful and Potentially Harmful Compounds (HPHC) in tobacco products to add PG/VG (and other things). FDA never issued a final updated list; it needs to finish the job now.
But it’s just rats
E-cigarette advocates will no doubt dismiss this work on the ground that rats are not people.
While one always has to be careful about extrapolating from animal studies to humans, the reality is that one simply cannot do such experiments on people. In addition, these (and other animal experiments) detected effects that no one has yet even looked for in the limited human studies of the effects of e-cigarettes on pregnancy outcomes.
Here are the authors’ key points:
- Rodent models have been a reliable and useful predictor of inhalation-induced harm to humans. These data indicate maternal use of Ecigs during pregnancy should not be considered safe, and begin to inform clinicians and women about potential long-term harm to their offspring.
- Exposure to electronic cigarettes (Ecigs) is known to increase risk factors for cardiovascular disease in both animals and humans.
- Maternal Ecig use during pregnancy in rodents is found to impair the vascular health of adolescent and adult offspring, but the critical gestation window for Ecig-induced vascular impairment is not known.
- This study demonstrates Ecig exposure during mid- and late-gestation (i.e. second or third trimester) results in impaired endothelial cell-mediated dilatation (i.e. middle cerebral artery reactivity) and alters anxiety-like behaviour in offspring.
- Maternal exposure prior to conception did not impact offspring’s vascular or anxiety-like behavioural outcomes.
Here is the abstract:
Studies have shown cerebrovascular dysfunction in offspring with full-gestational electronic cigarette (Ecig) exposure, but little is known about how individual trimester exposure impacts offspring health. This study aimed to determine if there is a critical window during gestation that contributes to vascular and anxiety-like behavioural changes seen with full-term exposure. To test this, rats were time-mated, and the pregnant dams were randomly assigned to Ecig exposure during first trimester (gestational day, GD2–7), second trimester (GD8–14), third trimester (GD15–21) or full-term gestation (GD2–21). We also assessed the effect of maternal preconception exposure. Both male and female offspring from all maternal exposure conditions were compared to offspring from dams under ambient air (control) conditions. Ecig exposure consisted of 60-puffs/day (5 days/week) using either 5 or 30 watts for each respective exposure group. We found that maternal exposure to Ecig in the second and third trimesters resulted in a decrease (23–38%) in vascular reactivity of the middle cerebral artery (MCA) reactivity in 3- and 6-month-old offspring compared to Air offspring. Further, the severity of impairment was comparable to the full-term exposure (31–46%). Offspring also displayed changes in body composition, body mass, anxiety-like behaviour and locomotor activity, indicating that Ecigs influence neurodevelopment and metabolism. Maternal preconception exposure showed no impact on offspring body mass, anxiety-like behaviour, or vascular function. Thus, the critical exposure window where Ecig affects vascular development in offspring occurs during mid- to late-gestation in pregnancy, and both 5 W and 30 W exposure produce significant vascular dysfunction compared to Air.
The full citation is: Mills A, Nassabeh S, Hurley A, Shouldis L, Chantler PD, Dakhlallah D, Olfert IM. Influence of gestational window on offspring vascular health in rodents with in utero exposure to electronic cigarettes. J Physiol. 2024 Aug 6. doi: 10.1113/JP286493. Epub ahead of print. PMID: 39106241. It is available here.